
In a significant development for Japan's healthcare advertising landscape, Google announced on November 21, 2024, that starting January 2025, licensed online pharmacies and telemedicine providers in Japan will be permitted to promote prescription drug services. This policy modification, outlined in Google's Healthcare and Medicines advertising guidelines, marks a substantial shift in how digital healthcare services can be marketed to Japanese consumers.
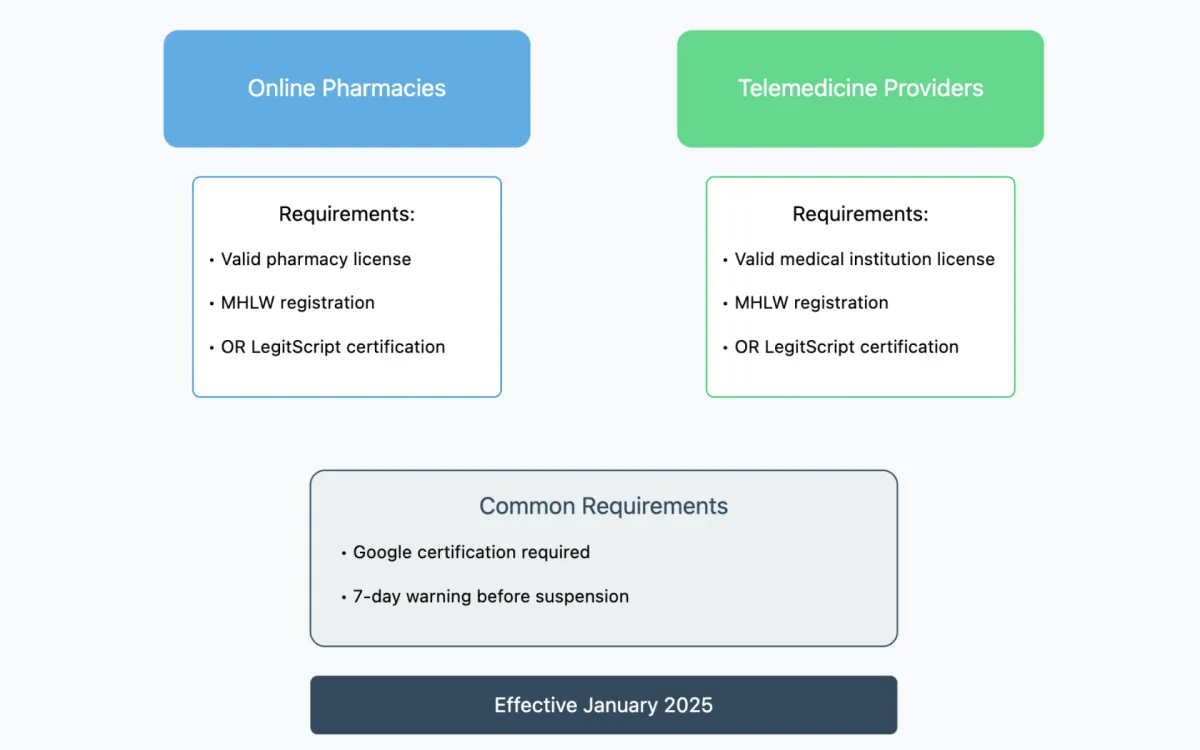
The new policy establishes specific requirements for healthcare providers seeking to advertise their services. According to the Ministry of Health, Labor, and Welfare (MHLW) guidelines, online medicine retailers must possess valid licensing and maintain current registration with the ministry. The framework creates two distinct pathways for qualification.
For online pharmacies, the authorization to promote prescription drug services requires either:
- A valid pharmacy license with active MHLW registration
- Certification through LegitScript's Healthcare Merchant Certification Program
Similarly, telemedicine providers must meet one of two criteria:
- Hold a valid medical institution license with MHLW registration
- Secure certification through LegitScript's Healthcare Merchant Certification Program
The implementation process includes several key procedural elements. According to Google's policy documentation, advertisers must complete a certification process directly with Google, beyond meeting the base regulatory requirements. This additional step ensures compliance with both governmental and platform-specific standards.
Consumer protection measures feature prominently in the policy framework. The guidelines incorporate a structured warning system for violations. Specifically, advertisers will receive at least seven days' notice before any account suspension, providing an opportunity to address compliance issues.
The policy maintains existing regulations for over-the-counter medicine retailers, focusing its updates specifically on prescription drug services. This selective approach demonstrates a calibrated expansion of digital healthcare advertising while preserving established consumer safeguards.
The historical context of this policy change reflects broader trends in Japanese healthcare delivery. Traditional restrictions on prescription drug advertising have long shaped the market, making this update particularly notable for its departure from previous norms.
Looking at enforcement mechanisms, the policy creates a multi-layered verification system. The dual-track qualification process, requiring either government licensing or third-party certification, establishes redundant safeguards for consumer protection. This approach aligns with Japan's traditionally careful regulation of healthcare services.
Technical implementation details indicate a structured rollout process. The policy launch will coincide with the opening of applications, suggesting a controlled transition period. This methodical approach allows for systematic verification of qualifications and gradual market adaptation.
Demographic implications are significant, considering Japan's aging population and increasing demand for accessible healthcare services. The policy potentially expands access to prescription services through digital channels, particularly relevant in remote areas or for mobility-restricted patients.
Market dynamics will likely shift as the policy takes effect. The ability to advertise prescription drug services could accelerate the adoption of telemedicine and online pharmacy services, potentially reshaping how Japanese consumers access healthcare.
Regulatory oversight remains robust under the new framework. The MHLW maintains its central role in licensing and registration, while LegitScript's certification program adds an additional layer of verification for service providers.
Key Facts
- Policy effective date: January 2025
- Announcement date: November 21, 2024
- Required certifications: MHLW license or LegitScript certification
- Warning period: Minimum 7 days before account suspension
- Scope: Prescription drug services for online pharmacies and telemedicine
- Current status: Applications will open upon policy launch
- Geographic application: Japan-specific policy update
- Oversight bodies: MHLW and LegitScript
- Verification requirement: Additional Google certification needed
- Impact on OTC medicines: No change to existing regulations

