Online advertisements for problematic peptides increased 208% in 2024 compared to 2023, while e-commerce marketplaces experienced a 276% growth rate in peptides-related product sales over the past five years, according to research published by LegitScript on December 10, 2025. The data quantifies how experimental peptides have proliferated across digital advertising platforms, social media channels, and e-commerce marketplaces despite their unapproved regulatory status.
The findings represent the first cross-platform dataset tracking problematic peptides activity across online advertisements, social media, and e-commerce channels simultaneously. Social media postings relating to problematic peptides sales increased 75% from 2023 to 2024. The company projects 2025 will surpass 2024's advertising volumes based on current trajectory.
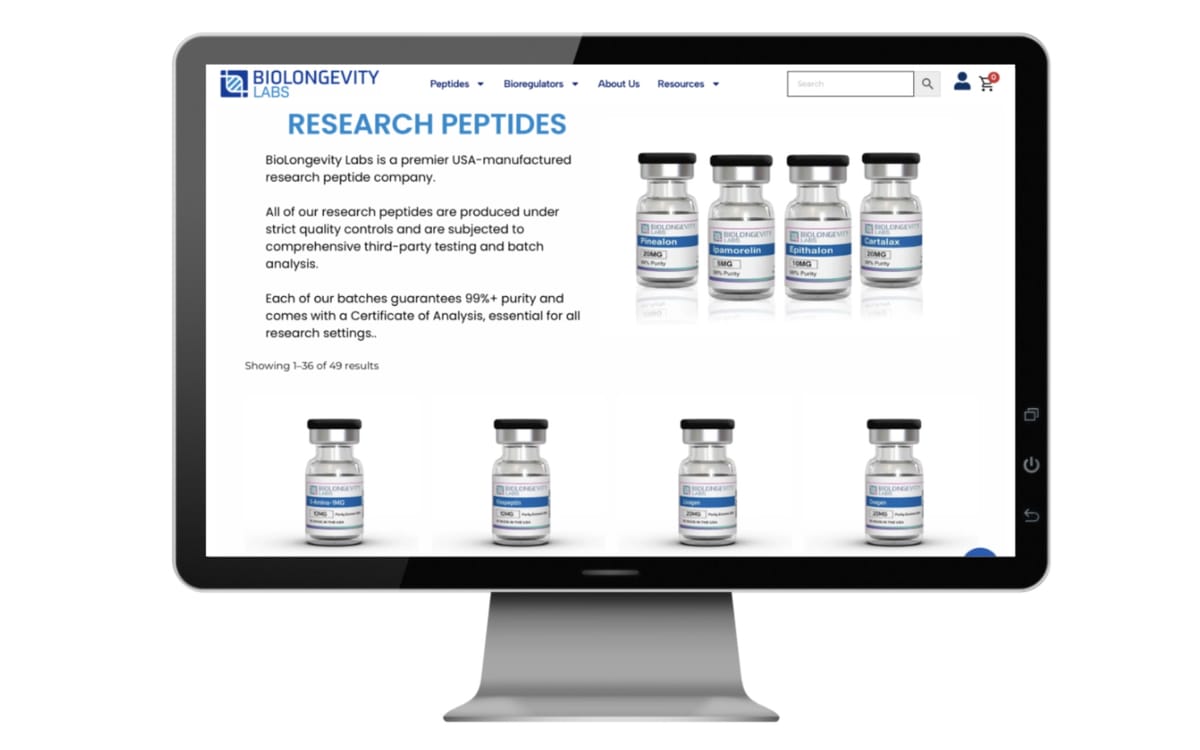
LegitScript's analysis tracks peptides marketed as "research chemicals" but intended for human consumption without FDA approval. These substances include compounds used for muscle growth, weight loss, tanning, sexual enhancement, and anti-aging purposes. The most frequently observed problematic products include Melanotan, BPC-157, TB-500, PT-141, and GLP-1 peptides.
Subscribe PPC Land newsletter ✉️ for similar stories like this one
"Peptides have quickly moved from a niche category into mainstream awareness, and that rise in popularity has been accompanied by a rise in unapproved products entering digital channels," said Gerard Olson, Director of Research at LegitScript. The company's multi-channel monitoring provides visibility that individual platforms and regulators lack by tracking advertising, sellers, payments, and enforcement behavior simultaneously.
The 2020-2025 dataset reveals acceleration patterns across all three distribution channels. Advertisement growth reached 678% when comparing 2024 to 2022, demonstrating exponential expansion rather than linear progression. The advertising surge reflects how sellers exploit digital platforms' scalability to reach broader audiences without traditional brick-and-mortar retail constraints.
E-commerce marketplace growth of 276% over five years indicates sustained seller migration toward online distribution. Marketplaces provide problematic sellers with established infrastructure, payment processing, and customer bases without requiring independent website development. This reduces barriers to entry for merchants selling experimental peptides through familiar shopping interfaces.
Social media's 75% year-over-year increase in peptides sales content demonstrates platform effectiveness for direct-to-consumer marketing. Sellers leverage social media's visual nature and influencer relationships to position unapproved substances alongside lifestyle content. Platforms' algorithmic amplification can accelerate visibility for content generating high engagement regardless of regulatory compliance.
The pharmaceutical advertising sector has faced increased platform scrutiny throughout 2025. Google launched restricted drug term certification requirements in July 2025, requiring approval for personalized targeting involving pharmaceutical terms in the United States, Canada, and New Zealand. The platform also blocked pill press equipment advertisementsstarting September 2025, targeting equipment that could facilitate illegal drug production.
Public interest in peptides has surged alongside the popularity of GLP-1 drugs like semaglutide, approved for type 2 diabetes and obesity treatment. This mainstream attention creates opportunities for sellers marketing unapproved peptides with similar claimed benefits but without regulatory oversight. Consumers often cannot distinguish between FDA-approved medications administered under medical supervision and experimental compounds sold online.
According to research published by the National Institutes of Health, 31 out of 370 drugs approved by the FDA between 2016 and 2023 were peptide-based. These legitimate pharmaceutical applications contrast sharply with the hundreds of unauthorized product names and terms LegitScript has observed across digital platforms. The divergence between approved medical applications and problematic online sales creates consumer confusion about regulatory status.
Merchants engaged in unauthorized peptide sales employ professional-looking websites claiming to offer high-purity, lab-tested products. Many build catalogs around compounds with strong consumer appeal tied to aesthetic, performance, or recovery outcomes. This positioning strategy borrows legitimacy cues from pharmaceutical contexts while avoiding regulatory compliance requirements.
"The business models used to market peptides, as well as the peptides themselves, are so varied that both consumers and businesses are easily confused by what is legitimate and what is not," said Olson. Product catalogs often highlight compounds tied to fitness, performance, or aesthetic goals, framed as safe and legal despite their unapproved status.
Common marketing patterns include "Not for Human Consumption" labeling, which sellers may use hoping to provide legal protection while actually indicating likely sale for unauthorized human use. Insufficient buyer-qualification steps allow purchases without professional researcher credentials or direct seller contact. Catalogs dominated by compounds frequently associated with misuse signal higher risk.
Buy ads on PPC Land. PPC Land has standard and native ad formats via major DSPs and ad platforms like Google Ads. Via an auction CPM, you can reach industry professionals.
Specific red flags include Melanotan 2 for tanning, Ipamorelin for muscle growth and healing, Tesamorelin for weight loss, CJC-1295 for performance enhancement, BPC-157 for injury recovery and anti-aging, Thymosin beta-4/TB-500 for muscle growth and injury recovery, and growth hormone releasing peptides. Marketplace enforcement mechanisms vary significantly across platforms, creating inconsistent regulatory environments.
FDA declared several peptides impermissible for compounding due to safety concerns, including BPC-157, CJC-1295, and Melanotan II. These substances cannot legally be compounded by pharmacies despite their availability through online channels. The regulatory gap between FDA prohibitions and online marketplace enforcement enables continued sales.
Payment processors and advertising platforms face mounting pressure to implement safeguards around sensitive medical content. Platform content moderation challenges affect enforcement consistency. Microsoft Advertising removed over one billion policy-violating advertisements in 2024, with common infractions involving misleading content and brand infringement.
"We're observing peptides follow a pattern we've seen in past online drug booms: rapid consumer interest, uneven product quality, and sellers moving faster than the safeguards meant to protect people," said Scott Roth, CEO of LegitScript. The company leverages the same research methodology used to navigate previous online pharmaceutical surges including GLP-1s and erectile dysfunction medications.
Problematic merchant websites vary in appearance depending on target demographics. Sellers offering broad peptide catalogs often favor clinical aesthetics with laboratory-reminiscent branding. Products typically appear as vials for injection, though some merchants offer nasal sprays or topical applications. Sites targeting bodybuilders may feature muscular imagery and performance-oriented language. Tanning-focused merchants display bronzed models and before-after skin tone photographs.
E-commerce marketplace policies continue evolving to address emerging product categories. Meta's Marketplace integrations with eBay and Poshmark demonstrate platform convergence in commerce, while regulatory frameworks struggle to keep pace with digital distribution innovation.
The Food and Drug Administration serves as the primary regulator of synthetic or processed peptides in the United States. Peptides intended for diagnosis, cure, mitigation, treatment, or prevention of disease are considered drugs under the Federal Food, Drug, and Cosmetic Act. Some peptide products may also be regulated as biological products under the Public Health Service Act.
FDA has issued warning letters and guidance documents related to synthetic peptides, including enforcement against unapproved sales and false or misleading claims. Even unapproved drugs fall under FDA purview. Compounded peptide drugs, created when pharmacists or physicians combine drug ingredients for specific patients, require regulatory compliance despite not undergoing pre-market FDA evaluation.
State boards of pharmacy regulate pharmacy practice in their jurisdictions, including licensing, dispensing, compounding, and scope of practice. Pharmacies and telemedicine companies prescribing or dispensing peptide drugs must adhere to laws in every jurisdiction where they are located and operate. This creates complex compliance requirements for online sellers serving national or international markets.
Peptides are short chains of amino acids serving as hormones, signaling molecules, and building blocks depending on their sequence and length. Some peptides like insulin occur naturally in the body, while others appear in food. Synthetically produced peptides serve research and medical purposes. Healthcare advertising faces unique regulatory challenges requiring specialized compliance expertise.
Legitimate peptide-based drugs prevent, treat, mitigate, or cure various disease conditions through replacing deficient peptides, guiding drugs to specific cells, or supporting research and diagnostics. Approved applications include bacterial infections, prostate cancer, HIV infections, cystic fibrosis, diabetes, and osteoporosis treatment. Administration methods include oral, transdermal, intravenous, subcutaneous, and nasal delivery.
Peptides in cosmetics and supplements claim potential health and cosmetic benefits including skin rejuvenation, joint function, and muscle recovery, though efficacy lacks substantial evidence for these applications. This creates market opportunities for unapproved products making unsubstantiated claims to consumers seeking performance or aesthetic improvements.
Experimental peptides sold online have not undergone proper FDA vetting and approval, including clinical trials for safety or efficacy and proper dosing studies. These products are not necessarily illegal to possess in research labs for legitimate professional study, but are illegal to market or sell for human consumption without FDA approval.
LegitScript's dataset provides platforms, e-commerce operators, payment providers, and the broader public with clarity into where high-risk peptides proliferate online and conditions enabling their growth. The company combines advanced AI-driven technology with domain expertise and curated market intelligence to help businesses stay ahead of emerging threats.
For payment companies and online platforms, the peptides surge creates compliance challenges around merchant monitoring and product certification. LegitScript offers merchant risk solutions helping payments companies assess risk and platform risk solutions helping internet platforms vet sellers and advertisers marketing various products.
Healthcare merchant certification provides recognition for businesses facilitating pharmaceutical sales through card-not-present transactions, considered high-risk by card networks. The certification process helps online pharmacies and healthcare merchants meet compliance and best practice standards while enabling payment processing and business promotion.
The marketing community faces brand safety concerns when advertisements appear alongside problematic peptides content on social media and e-commerce platforms. Platform monetization programs can inadvertently fund content promoting unapproved substances. AI-generated content proliferation creates additional challenges for distinguishing legitimate pharmaceutical information from promotional material for experimental compounds.
International regulatory divergence creates compliance complexities. The European Union's Digital Services Act establishes different requirements than US frameworks, forcing platforms to maintain multiple content moderation systems. Geographic variations in pharmaceutical advertising standards reflect cultural differences in acceptable promotional practices.
Content moderation infrastructure investments affect platform capabilities for identifying and restricting problematic listings. Platforms must balance open access for legitimate merchants with protection mechanisms preventing abuse and maintaining user trust. Effective enforcement requires coordination between automated systems and human expertise for complex cases requiring nuanced judgment.
The peptides proliferation demonstrates how digital platforms enable rapid market expansion for products operating in regulatory gray areas. Sellers exploit platform infrastructure while enforcement mechanisms lag behind distribution innovation. Cross-platform coordination remains limited, allowing merchants restricted on one platform to migrate to others with less stringent policies.
Subscribe PPC Land newsletter ✉️ for similar stories like this one
Timeline
- 2020-2025 – E-commerce marketplaces experience 276% growth rate in problematic peptides-related product sales
- 2022-2024 – Online advertisements for problematic peptides increase 678%
- 2023-2024 – Advertisements relating to problematic peptides increase 208%
- 2023-2024 – Social media postings relating to problematic peptides sales increase 75%
- July 2025 – Google launches restricted drug term certification for healthcare advertisers requiring approval for personalized pharmaceutical targeting
- July 2025 – Google blocks pill press equipment ads starting September to prevent equipment facilitating illegal drug production
- August 2025 – DeepIntent launches FAST solution for healthcare streaming ads with verified patient targeting
- October 2025 – Google revises prescription drug advertising policy for Canada, New Zealand, and United States markets
- December 10, 2025 – LegitScript releases primary research findings on problematic online peptides listings spanning 2020-2025
Subscribe PPC Land newsletter ✉️ for similar stories like this one
Summary
Who: LegitScript, a merchant and product certification and monitoring company, analyzed peptides activity across digital platforms. Payment processors, e-commerce operators, advertising platforms, and the general public receive new visibility into problematic peptides distribution patterns. Gerard Olson serves as Director of Research at LegitScript, while Scott Roth serves as CEO.
What: The first cross-platform dataset tracking problematic peptides revealed 208% growth in advertisements from 2023 to 2024, 276% growth in e-commerce marketplace sales over five years, and 75% increase in social media sales content year-over-year. The data covers unapproved peptides marketed as research chemicals but intended for human consumption, including Melanotan, BPC-157, TB-500, PT-141, and GLP-1 compounds.
When: LegitScript announced the findings on December 10, 2025, covering peptides activity from 2020 through 2025. The data shows acceleration patterns with 2025 projected to surpass 2024 advertising volumes. The research coincides with mainstream interest in peptide-based GLP-1 drugs and increased platform enforcement of pharmaceutical advertising policies throughout 2025.
Where: The problematic peptides proliferation spans online advertisements, social media platforms, and e-commerce marketplaces globally. LegitScript tracks activity across multiple channels simultaneously, providing visibility individual platforms and regulators lack. Sellers operate through professional-looking websites, marketplace storefronts, and social media channels reaching international audiences.
Why: Rapid consumer interest in peptides, uneven product quality, and sellers moving faster than protective safeguards enable the growth. Many merchants employ professional websites claiming high-purity products, blurring lines between legitimate research materials and unauthorized private consumption items. Consumers struggle distinguishing FDA-approved medications from experimental compounds, while platforms face challenges implementing consistent enforcement across varied business models and product categories.

